Hydrogen chloride
Covalent bonding at A'level
Cases where there isn't any difference from the simple view
If you stick closely
to modern A'level syllabuses, there is little need to move far from the
simple (GCSE) view. The only thing which must be changed is the
over-reliance on the concept of noble gas structures. Most of the
simple molecules you draw do in fact have all their atoms with noble gas
structures.
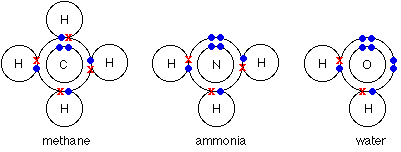
For example:
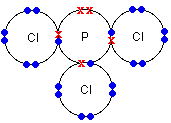
 Even with a more complicated molecule like PCl3, there's no problem. In this case, only the outer electrons are shown for simplicity. Each atom in this structure has inner layers of electrons of 2,8. Again, everything present has a noble gas structure.
Even with a more complicated molecule like PCl3, there's no problem. In this case, only the outer electrons are shown for simplicity. Each atom in this structure has inner layers of electrons of 2,8. Again, everything present has a noble gas structure.
 Cases where the simple view throws up problems
Cases where the simple view throws up problems
Comments
Post a Comment